
Hi Everybody!!
The highlight photos today are from 8-24-14. You may notice the birds against a golden background. The trees in the background are green as it is too early for any fall color. What causes the yellow glow? (Answer below!) So anyway, this morning I collected most of the feeders and carried them to the sink of bleach water to soak. I was just standing there brushing the containers to put away until next August. All of the sudden I had a flashback and saw my Mom standing at her kitchen sink, brushing her containers. I remember thinking at that time how old Mom was looking and frail. I asked her why she went through all the work to feed the hummer travelers year after year. Simply she said: We are supposed to help the creatures who come our way. Now I am the old lady standing at the sink washing 30 feeders! At least I know why I am doing it: We are supposed to. Enjoy!






https://en.wikipedia.org/wiki/Light
Light
From Wikipedia, the free encyclopedia
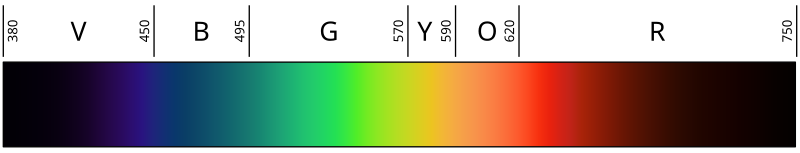
Light is radiant energy, usually referring to electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight.[1]Visible light is usually defined as having a wavelength in the range of 400 nanometres (nm), or 400×10−9 m, to 700 nanometres – between theinfrared, with longer wavelengths and the ultraviolet, with shorter wavelengths.[2][3] These numbers do not represent the absolute limits of human vision, but the approximate range within which most people can see reasonably well under most circumstances. Various sources define visible light as narrowly as 420 to 680[4][5] to as broadly as 380 to 800 nm.[6][7] Under ideal laboratory conditions, people can see infrared up to at least 1050 nm,[8] children and young adults ultraviolet down to about 310 to 313 nm.[9][10][11]
Primary properties of visible light are intensity, propagation direction, frequency or wavelength spectrum, and polarisation, while its speed in a vacuum, 299,792,458 meters per second, is one of the fundamental constants of nature. Visible light, as with all types of electromagnetic radiation (EMR), is experimentally found to always move at this speed in vacuum.[citation needed]
In common with all types of EMR, visible light is emitted and absorbed in tiny "packets" called photons, and exhibits properties of both waves andparticles. This property is referred to as the wave–particle duality. The study of light, known as optics, is an important research area in modern physics.
In physics, the term light sometimes refers to electromagnetic radiation of any wavelength, whether visible or not.[12][13] This article focuses on visible light. See the electromagnetic radiation article for the general term.

Electromagnetic spectrum and visible light
Main article: Electromagnetic spectrum
Generally, EM radiation, or EMR (the designation 'radiation' excludes static electric and magnetic and near fields) is classified by wavelength into radio, microwave, infrared, the visible region that we perceive as light, ultraviolet, X-rays and gamma rays.
The behaviour of EMR depends on its wavelength. Higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths. When EMR interacts with single atoms and molecules, its behaviour depends on the amount of energy per quantum it carries.
EMR in the visible light region consists of quanta (called photons) that are at the lower end of the energies that are capable of causing electronic excitation within molecules, which lead to changes in the bonding or chemistry of the molecule. At the lower end of the visible light spectrum, EMR becomes invisible to humans (infrared) because its photons no longer have enough individual energy to cause a lasting molecular change (a change in conformation) in the visual molecule retinal in the human retina, which change triggers the sensation of vision.
There exist animals that are sensitive to various types of infrared, but not by means of quantum-absorption. Infrared sensing in snakes depends on a kind of natural thermal imaging, in which tiny packets of cellular water are raised in temperature by the infrared radiation. EMR in this range causes molecular vibration and heating effects, which is how these animals detect it.
Above the range of visible light, ultraviolet light becomes invisible to humans, mostly because it is absorbed by the cornea below 360 nanometers and the internal lens below 400. Furthermore, the rods and cones located in the retina of the human eye cannot detect the very short (below 360 nm.) ultraviolet wavelengths, and are in fact damaged by ultraviolet. Many animals with eyes that do not require lenses (such as insects and shrimp) are able to detect ultraviolet, by quantum photon-absorption mechanisms, in much the same chemical way that humans detect visible light.
Electromagnetic theory as explanation for all types of visible light and all EM radiation
Main article: Electromagnetic radiation
In 1845, Michael Faraday discovered that the plane of polarisation of linearly polarised light is rotated when the light rays travel along the magnetic field direction in the presence of a transparent dielectric, an effect now known as Faraday rotation.[30] This was the first evidence that light was related to electromagnetism. In 1846 he speculated that light might be some form of disturbance propagating along magnetic field lines.[30] Faraday proposed in 1847 that light was a high-frequency electromagnetic vibration, which could propagate even in the absence of a medium such as the ether.
Faraday's work inspired James Clerk Maxwell to study electromagnetic radiation and light. Maxwell discovered that self-propagating electromagnetic waves would travel through space at a constant speed, which happened to be equal to the previously measured speed of light. From this, Maxwell concluded that light was a form of electromagnetic radiation: he first stated this result in 1862 in On Physical Lines of Force. In 1873, he published A Treatise on Electricity and Magnetism, which contained a full mathematical description of the behaviour of electric and magnetic fields, still known as Maxwell's equations. Soon after, Heinrich Hertz confirmed Maxwell's theory experimentally by generating and detecting radio waves in the laboratory, and demonstrating that these waves behaved exactly like visible light, exhibiting properties such as reflection, refraction, diffraction, and interference. Maxwell's theory and Hertz's experiments led directly to the development of modern radio, radar, television, electromagnetic imaging, and wireless communications.
In the quantum theory, photons are seen as wave packets of the waves described in the classical theory of Maxwell. The quantum theory was needed to explain effects even with visual light that Maxwell's classical theory could not (such as spectral lines).
(Please see link for complete Page)
(Please see link for complete Page)
















...this is brendasue signing off from Rainbow Creek. See you next time!

O+O



No comments:
Post a Comment